Does air pressure vary with respect to air temperature?
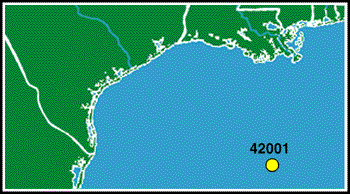
Lets examine some air pressure data from the Gulf of Mexico. The following data was measured at Station 42001 in the central Gulf. In January, when air temperatures average 20.7 C (69F), the average pressure is 1019.1 mb. In June, when the average air temperature is 27.6C (81.7F), the average air pressure is 1015.0 mb. As you can see, air pressure does vary according to temperature. Cold air is more dense than warm air, i.e., it weighs more. As a result, it tends to sink. Warm air, on the other hand, is less dense. Therefore, it weighs less and tends to rise. Meteorologists say that warm air is buoyant.
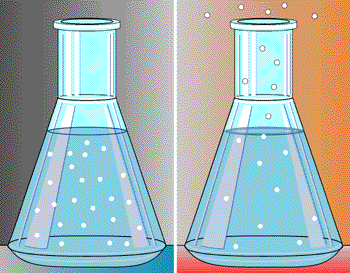
Look at the above image. Imagine that the little circles in the beakers are air molecules. The image on the left shows the beaker of molecules when the air is cool. However, the image on the right shows the same beaker when it is warmed. As you can see, some of the molecules leave the beaker when heat is applied. The same situation occurs when the atmosphere is heated: some of the air molecules "leave" or float away, thus causing decreased air pressure.
Return to Science Education Home



